2.7. Linear Rigid Rotor#
Overview#
This section covers the quantum mechanical treatment of a linear rigid rotor (e.g., a diatomic molecule) and shows how to derive thermodynamic properties from its rotational partition function.
Review of the Linear Rigid Rotor#
Show code cell source
import numpy as np
import matplotlib.pyplot as plt
from mpl_toolkits.mplot3d.art3d import Poly3DCollection
from scipy.constants import k, eV
from labellines import labelLines
from myst_nb import glue
fig, axs = plt.subplot_mosaic([[0]], figsize=(4, 4))
# Plot the energy levels (blue lines)
for J in range(0, 3):
g_J = 2 * J + 1
x_min = -0.04 - 0.1 * (g_J - 1) / 2
for i in range(g_J):
# Plot each degenerate sub-level horizontally
if i == (g_J - 1) / 2:
energy_line = axs[0].plot(
[x_min, x_min + 0.08],
[J * (J + 1), J * (J + 1)],
color='blue',
label=r'$E_{%d}$' % J
)
else:
axs[0].plot(
[x_min, x_min + 0.08],
[J * (J + 1), J * (J + 1)],
color='blue'
)
x_min += 0.1
# Label only one line at each J for clarity
labelLines(energy_line, zorder=2.5)
axs[0].set_ylabel('Energy (arb. units)')
axs[0].set_xticks([])
axs[0].set_yticks([])
axs[0].spines['top'].set_visible(False)
axs[0].spines['bottom'].set_visible(False)
axs[0].spines['right'].set_visible(False)
axs[0].spines['left'].set_visible(False)
plt.tight_layout()
glue('linear_rigid_rotor_energy_levels', fig, display=False)
plt.close(fig)
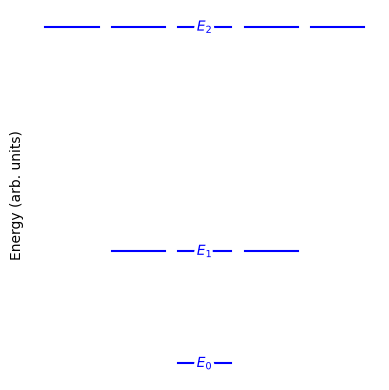
Fig. 21 Energy levels for a linear rigid rotor. The level labeled \(E_J\) has degeneracy \(g_J = 2J + 1\). For example, the \(J=2\) level is split into five degenerate microstates corresponding to \(m = -2, -1, 0, 1, 2\).#
For a rigid, linear rotor of moment of inertia \(I\), the energy levels are:
Here, \(\hbar\) is the reduced Planck constant, and \(g_J = 2J + 1\) is the degeneracy of level \(E_J\).
Physical Significance of the Moment of Inertia
The moment of inertia \(I\) measures how mass is distributed around the rotation axis. For a diatomic molecule of atoms A and B,
where \(r\) is the bond length and \(\mu\) is the reduced mass of the two atoms.
Partition Function for a Linear Rigid Rotor#
In the canonical ensemble, the rotational partition function is:
Summation vs. Levels
Sum over \(i\): sums over individual microstates (each degenerate sub-level).
Sum over \(J\): reorganizes the sum by energy level \(E_J\), factoring in the degeneracy \(g_J = 2J+1\).
High-Temperature Approximation#
When \(k_\mathrm{B} T \gg \frac{\hbar^2}{2I}\), we can approximate the discrete sum by converting it into an integral. Let us set
Hence,
Evaluating this integral,
We define the rotational temperature \(\Theta_{\mathrm{rot}}\) by
Thus, for a heteronuclear diatomic rotor (symmetry factor \(\sigma = 1\)),
Symmetry Factor \(\sigma\)
For homonuclear diatomics, or other symmetric linear rotors, identical orientations in space might be indistinguishable, leading to \(\sigma=2\). This modifies the partition function to
Whether or not you include \(\sigma\) depends on the level of detail needed (e.g., for absolute entropy calculations).
Ensemble Averages#
Natural Logarithm of the Partition Function#
From the high-\(T\) approximation (with \(\sigma=1\) for simplicity),
Internal Energy#
The (rotational) internal energy \(U_{\mathrm{rot}}\) is given by
Since \(\ln q_{\mathrm{rot}} = \ln T - \ln \Theta_{\mathrm{rot}},\) we get
Hence,
Heat Capacity at Constant Volume#
The rotational contribution to the heat capacity is
Physically, this means one linear rotor contributes \(k_{\mathrm{B}}\) to the heat capacity in the classical (high-\(T\)) limit, corresponding to its two rotational degrees of freedom (each contributes \(\tfrac{1}{2}k_{\mathrm{B}}\)).
Note
In a more rigorous quantum treatment (and for lower temperatures), the partition function and the resulting averages must use the full sum over \(J\). At sufficiently low \(T\), only the \(J=0\) and \(J=1\) levels are significantly populated, which reduces the effective heat capacity below \(k_\mathrm{B}\).
