1.4. Real Gases#
Overview#
Under high pressures, high densities, or low temperatures, we cannot treat gases as sets of non-interacting point particles. Higher pressures imply more frequent collisions and higher densities, higher densities are associated with closer molecules that can more strongly interact and experience each other’s finite size, and lower temperatures indicate that molecules have lower kinetic energy and are more disposed to be affected by these interactions. This section presents approaches for quantifying deviations from ideal behavior and the van der Waals equation of state, which is one of the simplest models for real gases and the subject of the Nobel Prize in Physics 1910.
Deviations from Ideal Behavior#
Compressibility Factor#
Show code cell source
import matplotlib.pyplot as plt
import numpy as np
import pandas as pd
from scipy.constants import R
from labellines import labelLines
from myst_nb import glue
from scipy.interpolate import interp1d
# Load the experimental data for water at 300 K
df = pd.read_csv("../_static/section-04/isothermal-properties-for-water-T=300.csv").iloc[:, [0, 1, 2, 3]]
df.columns = ["T", "P", "rho_m", "V_m"] # Rename columns
# Calculate the compressibility factor
df["P_Pa"] = df["P"] * 1e5 # Convert pressure from bar to Pa
df["Z"] = df["P_Pa"] * df["V_m"] / (R * df["T"])
# Set up figure and axis
fig, ax = plt.subplots(figsize=(4, 4))
ax.set_xlim(0, 1000)
ax.set_ylim(0, 2)
ax.set_xlabel("Pressure (bar)")
ax.set_ylabel(r"$Z$")
ax.grid(True, linestyle="--", alpha=0.7)
# Plot experimental compressibility factor
data_line = ax.plot(df["P"], df["Z"], "b-", label="$Z_\\text{water}$")
labelLines(data_line, xvals=[400], zorder=2.5)
# Plot ideal gas line
x_values = np.linspace(0, 1000, 100)
ideal_line = ax.plot(x_values, np.ones_like(x_values), "r--", label="$Z_\\text{ideal}$")
labelLines(ideal_line, xvals=[400], zorder=2.5)
# Interpolate to find intersection with ideal gas line
interp_Z = interp1d(df["P"], df["Z"], kind='linear', fill_value="extrapolate")
intersection_pressure = interp1d(df["Z"] - 1, df["P"], kind='linear')(0)
intersection_Z = interp_Z(intersection_pressure)
# Find pressure and Z value at minimum Z
min_Z_index = df["Z"].idxmin()
min_pressure = df.loc[min_Z_index, "P"]
min_Z = df.loc[min_Z_index, "Z"]
# Annotate ideal and coincidentally ideal points
ax.annotate("Ideal",
xy=(0, 1), xytext=(200, 1.25),
arrowprops=dict(arrowstyle="->"),
ha='center', va='center', zorder=4)
ax.annotate("Coincidentally\nideal",
xy=(intersection_pressure, 1), xytext=(800, 0.75),
arrowprops=dict(arrowstyle="->"),
ha='center', va='center', zorder=4)
# Draw vertical lines for reference
ax.axvline(x=min_pressure, color="black", linestyle=":")
ax.axvline(x=intersection_pressure, color="black", linestyle=":")
# Highlight regions of attractive and repulsive behavior
ax.fill_between([min_pressure, intersection_pressure], 0, 2, color="magenta", alpha=0.2, label="Attractive")
ax.fill_between([intersection_pressure, 1000], 0, 2, color="gray", alpha=0.2, label="Repulsive")
# Annotate regions
x_values_for_attractive = np.linspace(min_pressure, intersection_pressure, 100)
attractive_line = ax.plot(x_values_for_attractive, np.ones_like(x_values_for_attractive) * min_Z, "m-", label="Attractive", alpha=0.0)
labelLines(attractive_line, xvals=np.mean([min_pressure, intersection_pressure]), zorder=2.5)
x_values_for_repulsive = np.linspace(intersection_pressure, 1000, 100)
repulsive_line = ax.plot(x_values_for_repulsive, np.ones_like(x_values_for_repulsive) * min_Z, "gray", label="Repulsive", alpha=0.0)
labelLines(repulsive_line, xvals=800, zorder=2.5)
# Adjust layout and display
plt.tight_layout()
glue('compressibility-factor', fig, display=False)
plt.close(fig)
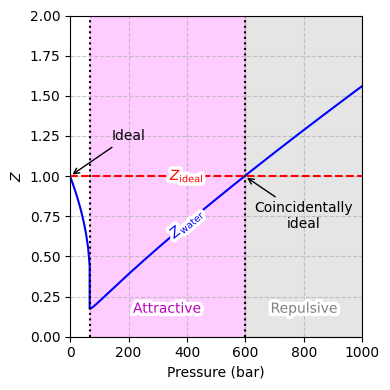
Fig. 8 Compressibility factor \(Z\) of water at 300 K as a function of pressure, with the ideal-gas line (\(Z=1\)) included for comparison. The dip below \(Z=1\) highlights net attractive forces (most pronounced at the minimum \(Z\)), while crossing above \(Z=1\) at higher pressures reflects repulsive interactions. The point at which the real-gas curve intersects the ideal-gas line indicates a coincidental match to ideal behavior.#
One of the ways to quantify deviations from ideal behavior is by way of the compressibility factor \(Z\):
Pressure |
\(Z\) |
Volume |
Analysis |
Water |
|---|---|---|---|---|
High |
\(> 1\) |
\(V_\text{real} > V_\text{ideal}\) |
Repulsive interactions |
Pauli repulsion (steric hindrance) |
Medium |
\(< 1\) |
\(V_\text{real} < V_\text{ideal}\) |
Attractive interactions |
H bonding and van der Waals interactions |
Low |
\(= 1\) |
\(V_\text{real} = V_\text{ideal}\) |
Ideal gas behavior |
Tip
Can you demonstrate why \(V_\text{real} > V_\text{ideal}\) for \(Z > 1\) and \(V_\text{real} < V_\text{ideal}\) for \(Z < 1\)?
Tip
What might be the cause of attractive interactions in a gas of carbon dioxide or methane molecules?
Van der Waals Fluid#
Van der Waals Equation of State#
The van der Waals equation of state is one of the simplest models for real gases and the subject of the Nobel Prize in Physics 1910:
Extensive Volume |
Intensive Volume |
|
|---|---|---|
Per Particle |
\[\left( P + \frac{a_\text{p} N^2}{V^2} \right) \left( V - b_\text{p} N \right) = N k_\text{B} T\]
|
\[\left( P + \frac{a_\text{p}}{V_\text{p}^2} \right) \left( V_\text{p} - b_\text{p} \right) = k_\text{B} T\]
|
Per Mole |
\[\left( P + \frac{a_\text{m} n^2}{V^2} \right) \left( V - b_\text{m} n \right) = n R T\]
|
(8)#\[\left( P + \frac{a_\text{m}}{V_\text{m}^2} \right) \left( V_\text{m} - b_\text{m} \right) = R T,\]
|
where \(a\) and \(b\) are constants that quantify two deviations from ideal behavior:
\(a\): Attractive interactions between particles.
\(b\): Volume occupied by the particles.
Van der Waals Isotherm#
Show code cell source
import matplotlib.pyplot as plt
from matplotlib.lines import Line2D
import numpy as np
import pandas as pd
from scipy.constants import R
from labellines import labelLines
from myst_nb import glue
# van der Waals constants for carbon dioxide
a_m_L_bar = 3.6551 # L^2 bar / mol^2
b_m_L = 0.042816 # L / mol
L_to_m3 = 1e-3 # m^3 / L
bar_to_Pa = 1e5 # Pa / bar
a_m = a_m_L_bar * L_to_m3**2 * bar_to_Pa # m^6 Pa / mol^2
b_m = b_m_L * L_to_m3 # m^3 / mol
# van der Waals critical properties for carbon dioxide
Tc_vdW = 8 * a_m / (27 * b_m * R) # K
Pc_vdW = a_m / (27 * b_m**2) # Pa
Vc_vdW = 3 * b_m # m^3 / mol
# Experimental phase change data for carbon dioxide
Tc_exp = 304.18 # K
Pc_exp = 73.80 * bar_to_Pa # Pa
Vc_exp = 0.0919 * L_to_m3 # m^3 / mol
# van der Waals pressure function
def van_der_waals_pressure(a, b, T, V):
return R * T / (V - b) - a / V**2
# Load experimental data
def load_experimental_data(filename):
df = pd.read_csv(filename).iloc[:, [0, 1, 2, 3]]
df.columns = ["Tc", "P", "rho_m", "V_m"]
return df
df_lt_Tc = load_experimental_data("../_static/section-04/isothermal-properties-for-carbon-dioxide-Tr=0.9.csv")
df_at_Tc = load_experimental_data("../_static/section-04/isothermal-properties-for-carbon-dioxide-Tr=1.0.csv")
df_gt_Tc = load_experimental_data("../_static/section-04/isothermal-properties-for-carbon-dioxide-Tr=1.1.csv")
# Set up figure
fig, ax = plt.subplots(figsize=(4, 4))
Vm_vdW = np.linspace(0.5 * Vc_vdW, 20 * Vc_vdW, 1000)
# Plot van der Waals isotherms
colors = ["b", "r", "m"]
temps = [0.9 * Tc_vdW, Tc_vdW, 1.1 * Tc_vdW]
labels = ["$T < T_c$", "$T = T_c$", "$T > T_c$"]
lines = []
for T, color, label in zip(temps, colors, labels):
P_vdW = van_der_waals_pressure(a_m, b_m, T, Vm_vdW)
line = ax.plot(Vm_vdW / L_to_m3, P_vdW / bar_to_Pa, color + "-", label=label)
lines.append(line)
labelLines(line, xvals=[0.3], zorder=2.5)
# Plot experimental isotherms
for df, color in zip([df_lt_Tc, df_at_Tc, df_gt_Tc], colors):
ax.plot(df["V_m"] / L_to_m3, df["P"], color + "-", alpha=0.5)
# Legend and labels
legend_elements = [Line2D([0], [0], c='k', label='van der Waals', ls="-"),
Line2D([0], [0], c='k', label='Experimental', ls="-", alpha=0.5)]
ax.set_xlim(0, 1)
ax.set_ylim(0, 100)
ax.set_xlabel("Molar Volume (L/mol)")
ax.set_ylabel("Pressure (bar)")
ax.legend(handles=legend_elements, loc='lower left')
ax.grid(True, linestyle="--", alpha=0.7)
# Adjust layout and display
plt.tight_layout()
glue('van-der-waals-isotherms', fig, display=False)
plt.close(fig)
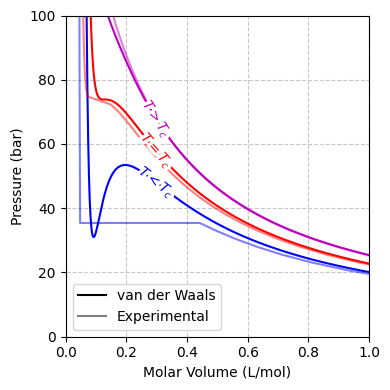
Fig. 9 Comparison of experimental and van der Waals isotherms for CO2 at temperatures below, at, and above the critical temperature \(T_c\). The subcritical isotherm (\(T < T_c\)) displays the characteristic loop associated with phase separation, while the isotherm at \(T = T_c\) shows an inflection point, and supercritical conditions (\(T > T_c\)) no longer exhibit a liquid–vapor transition.#
Critical Point#
Show code cell source
# Reference: https://scipython.com/blog/the-maxwell-construction/
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
from matplotlib.lines import Line2D
from scipy.constants import R as R_SI
from scipy.integrate import quad
from scipy.optimize import curve_fit, newton
from scipy.signal import argrelextrema
from scipy import stats
from labellines import labelLines
from myst_nb import glue
R = R_SI / 100 # Gas constant in L bar / mol K
# van der Waals constants for carbon dioxide
a_m = 3.6551 # L^2 bar/mol^2
b_m = 0.042816 # L/mol
# van der Waals critical properties for carbon dioxide
Tc_vdW = 8 * a_m / (27 * b_m * R) # K
Pc_vdW = a_m / (27 * b_m**2) # bar
Vc_vdW = 3 * b_m # L/mol
# Reduced-pressure form of the van der Waals equation of state
def vdw(Tr, Vr):
"""Van der Waals equation of state.
Return the reduced pressure from the reduced temperature and volume.
"""
pr = 8*Tr/(3*Vr-1) - 3/Vr**2
return pr
# Maxwell construction of the reduced-pressure form of the van der Waals equation of state
def vdw_maxwell(Tr, Vr):
"""Van der Waals equation of state with Maxwell construction.
Return the reduced pressure from the reduced temperature and volume,
applying the Maxwell construction correction to the unphysical region
if necessary.
"""
pr = vdw(Tr, Vr)
if Tr >= 1:
# No unphysical region above the critical temperature.
return pr
if min(pr) < 0:
raise ValueError('Negative pressure results from van der Waals'
' equation of state with Tr = {} K.'.format(Tr))
# Initial guess for the position of the Maxwell construction line:
# the volume corresponding to the mean pressure between the minimum and
# maximum in reduced pressure, pr.
iprmin = argrelextrema(pr, np.less)
iprmax = argrelextrema(pr, np.greater)
Vr0 = np.mean([Vr[iprmin], Vr[iprmax]])
def get_Vlims(pr0):
"""Solve the inverted van der Waals equation for reduced volume.
Return the lowest and highest reduced volumes such that the reduced
pressure is pr0. It only makes sense to call this function for
T<Tc, ie below the critical temperature where there are three roots.
"""
eos = np.poly1d( (3*pr0, -(pr0+8*Tr), 9, -3) )
roots = eos.r
roots.sort()
Vrmin, _, Vrmax = roots
return Vrmin, Vrmax
def get_area_difference(Vr0):
"""Return the difference in areas of the van der Waals loops.
Return the difference between the areas of the loops from Vr0 to Vrmax
and from Vrmin to Vr0 where the reduced pressure from the van der Waals
equation is the same at Vrmin, Vr0 and Vrmax. This difference is zero
when the straight line joining Vrmin and Vrmax at pr0 is the Maxwell
construction.
"""
pr0 = vdw(Tr, Vr0)
Vrmin, Vrmax = get_Vlims(pr0)
return quad(lambda vr: vdw(Tr, vr) - pr0, Vrmin, Vrmax)[0]
# Root finding by Newton's method determines Vr0 corresponding to
# equal loop areas for the Maxwell construction.
Vr0 = newton(get_area_difference, Vr0)
pr0 = vdw(Tr, Vr0)
Vrmin, Vrmax = get_Vlims(pr0)
# Set the pressure in the Maxwell construction region to constant pr0.
pr[(Vr >= Vrmin) & (Vr <= Vrmax)] = pr0
return pr, Vrmin, Vrmax
# Reduced volume range
Vr = np.linspace(0.5, 20, 1000)
fig, axs = plt.subplot_mosaic([[0, 1]], figsize=(8, 4), sharex=True, sharey=True)
# Plot the van der Waals isotherm below the critical temperature
line1 = axs[0].plot(Vr * Vc_vdW,
vdw(0.9, Vr) * Pc_vdW,
'b-', label='$T = 0.9 T_c$')
labelLines(line1, xvals=0.4, zorder=2.5)
Pr_max, Vr_liq, Vr_vap = vdw_maxwell(0.9, Vr)
Pr_equ = Pr_max[np.argwhere(np.diff(Pr_max) == 0).flatten().mean().astype(int)]
Vr_int = 0.140076 / Vc_vdW # https://www.wolframalpha.com/input?i=V%5E3-%28b%2BR*T%2FP%29*V%5E2%2Ba%2FP*V-a*b%2FP%3D0+for+a%3D3.6551%2C+b%3D0.042816%2C+R%3D8.314462618%2F100%2C+T%3D0.9*8*a%2F%2827*b*R%29%2C+P%3D0.64699835*a%2F%2827*b%5E2%29
# print(f"At Tr = 0.90, the molar volume of the liquid phase is {Vr_liq * Vc_vdW:.4f} L/mol, the molar volume of the vapor phase is {Vr_vap * Vc_vdW:.4f} L/mol, and the equilibrium pressure is {Pr_equ * Pc_vdW:.4f} bar.")
# Calculate the molar volume of the liquid and vapor phases at Tr = 0.85
Pr_max_85, Vr_liq_85, Vr_vap_85 = vdw_maxwell(0.85, Vr)
Pr_equ_85 = Pr_max_85[np.argwhere(np.diff(Pr_max_85) == 0).flatten().mean().astype(int)]
# print(f"At Tr = 0.85, the molar volume of the liquid phase is {Vr_liq_85 * Vc_vdW:.4f} L/mol, the molar volume of the vapor phase is {Vr_vap_85 * Vc_vdW:.4f} L/mol, and the equilibrium pressure is {Pr_equ_85 * Pc_vdW:.4f} bar.")
# Calculate the molar volume of the liquid and vapor phases at Tr = 0.8
# Plot the van der Waals isotherm at the critical temperature
line2 = axs[0].plot(Vr * Vc_vdW,
vdw(1.0, Vr) * Pc_vdW,
'r-', label='$T = T_c$')
labelLines(line2, xvals=0.4, zorder=2.5)
# Fill the Maxwell construction region
Vr_vals_fill_l = np.linspace(Vr_liq, Vr_int, 100) # Range of volumes for filling
Pr_vals_fill_l = vdw(0.9, Vr_vals_fill_l) * Pc_vdW # Calculate pressures along the curve
Vr_vals_fill_r = np.linspace(Vr_int, Vr_vap, 100) # Range of volumes for filling
Pr_vals_fill_r = vdw(0.9, Vr_vals_fill_r) * Pc_vdW # Calculate pressures along the curve
# Fill the area between the vdw curve and the phase transition pressure Pr_equ
axs[0].fill_between(
Vr_vals_fill_l * Vc_vdW, # Scaled volume
Pr_vals_fill_l, # Pressure from vdw curve
np.ones_like(Vr_vals_fill_l) * Pr_equ * Pc_vdW, # Constant transition pressure
color='blue', # Styling for the fill
)
axs[0].fill_between(
Vr_vals_fill_r * Vc_vdW, # Scaled volume
Pr_vals_fill_r, # Pressure from vdw curve
np.ones_like(Vr_vals_fill_r) * Pr_equ * Pc_vdW, # Constant transition pressure
color='blue', # Styling for the fill
)
# Plot the molar volume of the liquid and vapor phases at Tr = 0.9
axs[0].plot(Vr_liq * Vc_vdW, Pr_equ * Pc_vdW, 'wo', mec='b')
axs[0].plot(Vr_vap * Vc_vdW, Pr_equ * Pc_vdW, 'wo', mec='b')
# Annotate the Maxwell construction
x_maxwell = np.mean([Vc_vdW, 0.3])
axs[0].annotate("Maxwell\nconstruction",
xy=(x_maxwell, Pr_equ * Pc_vdW),
xytext=(x_maxwell, 40),
arrowprops=dict(arrowstyle="->"),
ha='center', va='center',
zorder=4)
# Plot and annotate the critical point
axs[0].plot(Vc_vdW, Pc_vdW, 'wo', mec='r')
Vm_vals_Vc = np.linspace(1e-3, 0.5, 100)
y_supercritical = np.mean([Pc_vdW, 80])
line3 = axs[0].plot(Vm_vals_Vc, np.ones_like(Vm_vals_Vc) * y_supercritical, 'r-', alpha=0, label='$V_{\\text{m},c}$')
labelLines(line3, xvals=Vc_vdW, zorder=2.5)
Vm_vals_Pc = np.linspace(0, 0.5, 100)
line4 = axs[0].plot(Vm_vals_Pc, np.ones_like(Vm_vals_Pc) * Pc_vdW, 'r-', alpha=0, label='$P_c$')
labelLines(line4, xvals=0.035, zorder=2.5)
axs[0].axvline(Vc_vdW, color='r', linestyle=':', zorder=-200)
axs[0].axhline(Pc_vdW, color='r', linestyle=':', zorder=-200)
# Approximate the coexistence curve with a function
# Define the functional form: A * ln(B * Vm) * exp(-C * Vm ** 0.25)
def fit_function(Vm, A, B, C):
return A * np.log(B * Vm) * np.exp(-C * Vm ** 0.25)
# Extract data points: (Vm_liq, P_eq), (Vc, Pc), (Vm_vap, P_eq)
Vm_data = np.array([Vr_liq * Vc_vdW,
Vr_liq_85 * Vc_vdW,
Vc_vdW,
Vr_vap_85 * Vc_vdW,
Vr_vap * Vc_vdW])
P_data = np.array([Pr_equ * Pc_vdW,
Pr_equ_85 * Pc_vdW,
Pc_vdW,
Pr_equ_85 * Pc_vdW,
Pr_equ * Pc_vdW])
# Provide an initial guess for the parameters A, B, C
initial_guess = [100, 10, 1]
# Fit the curve to the data
params, covariance = curve_fit(fit_function, Vm_data, P_data, p0=initial_guess)
# Extract fitted parameters
A_fit, B_fit, C_fit = params
# print(f"Fitted parameters: A = {A_fit}, B = {B_fit}, C = {C_fit}")
# Generate data for plotting the fitted curve
P_fit = fit_function(Vm_vals_Vc, A_fit, B_fit, C_fit)
# Fitted curve
axs[0].fill_between(Vm_vals_Vc, P_fit, 0, color='magenta', alpha=0.2, zorder=-100)
axs[1].fill_between(Vm_vals_Vc, P_fit, 0, color='magenta', alpha=0.2, zorder=-100)
# Annotate the coexistence curve
line5 = axs[1].plot(Vm_vals_Vc - 0.04, P_fit, 'b-', label='Liquid', alpha=0)
labelLines(line5, xvals=0.04, zorder=2.5)
axs[1].fill_between(Vm_vals_Vc[Vm_vals_Vc <= Vc_vdW],
np.ones_like(Vm_vals_Vc[Vm_vals_Vc <= Vc_vdW]) * Pc_vdW,
P_fit[Vm_vals_Vc <= Vc_vdW],
color='blue',
alpha=0.2)
line6 = axs[1].plot(Vm_vals_Vc + 0.04, P_fit, 'r-', label='Vapor', alpha=0)
labelLines(line6, xvals=0.32, zorder=2.5)
axs[1].fill_between(Vm_vals_Vc[Vm_vals_Vc >= Vc_vdW],
np.ones_like(Vm_vals_Vc[Vm_vals_Vc >= Vc_vdW]) * Pc_vdW,
P_fit[Vm_vals_Vc >= Vc_vdW],
color='red',
alpha=0.2)
line7 = axs[1].plot(Vm_vals_Pc, np.ones_like(Vm_vals_Pc) * y_supercritical, '-', c="gray", alpha=0, label='Supercritical fluid')
labelLines(line7, xvals=Vc_vdW, zorder=2.5)
axs[1].fill_between(Vm_vals_Pc,
np.ones_like(Vm_vals_Pc) * Pc_vdW,
80,
color='gray',
alpha=0.2)
line8 = axs[1].plot(Vm_vals_Pc, np.ones_like(Vm_vals_Pc) * 40, 'm-', alpha=0, label='Liquid-vapor\nmixture')
labelLines(line8, xvals=x_maxwell, zorder=2.5)
axs[0].set_xlim(0, 0.5)
axs[0].set_ylim(30, 80)
axs[0].set_xlabel("Molar Volume (L/mol)")
axs[1].set_xlabel("Molar Volume (L/mol)")
axs[0].set_ylabel("Pressure (bar)")
axs[0].grid(True, linestyle="--", alpha=0.7)
# Adjust layout and display
plt.tight_layout()
glue('van-der-waals-critical-point', fig, display=False)
plt.close(fig)
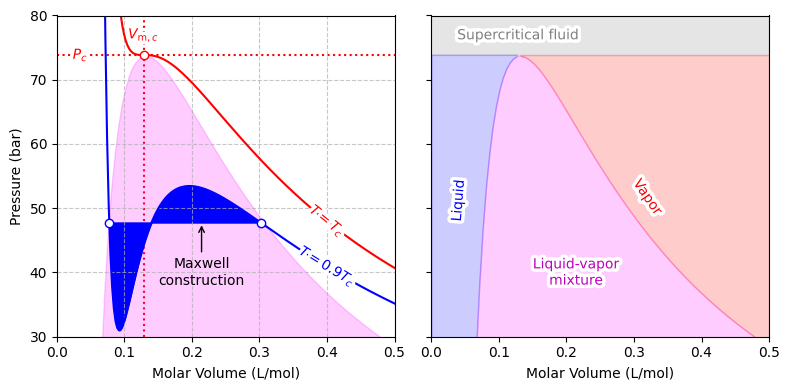
Fig. 10 Van der Waals isotherm for CO2 at \(T = 0.9\,T_c\), with the Maxwell construction illustrating how the unphysical loop is replaced by a horizontal tie line defining liquid–vapor coexistence. The critical point at \(T = T_c\) is also shown, where the liquid and vapor phases merge.#
Expanding Equation (8) and rearranging terms:
Complete Derivation of the Cubic Equation in \(V_\text{m}\)
1. Expand Equation (8) and Subtract \(RT\) From Both Sides:
2. Multiply Both Sides by \(V_\text{m}^2 / P\):
3. Group Terms:
At the critical point, Equation (9) simplifies to:
Comparing these equations:
Complete Derivation of the Critical Point
1. Set Coefficients Equal to Each Other and Solve for Powers of \(V_{\text{m},c}\):
2. Divide \(V_{\text{m},c}^3\) by \(V_{\text{m},c}^2\) and Eliminate \(P_{\text{c}}\):
3. Substitute This Result Into \(V_{\text{m},c}^3\) and Solve for \(P_{\text{c}}\):
4. Substitute These Results Into \(V_{\text{m},c}\) and Solve for \(T_{\text{c}}\):
Corresponding States#
Show code cell source
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
from matplotlib.lines import Line2D
from labellines import labelLines
from scipy.constants import R
from myst_nb import glue
# Define styles for species
style_for_species = {"Ar": 'o', "O2": '^', "H2O": 's', "CO2": 'p'}
label_for_species = {"Ar": "Ar", "O2": "O$_2$", "H2O": "H$_2$O", "CO2": "CO$_2$"}
line_style_for_species = {"Ar": "-", "O2": "--", "H2O": "-.", "CO2": ":"}
color_for_temperature = {0.9: "blue", 1.0: "blue", 1.1: "red", 1.2: "magenta", 2.0: "red"}
color_for_species = {"Ar": "black", "O2": "blue", "H2O": "red", "CO2": "magenta"}
# Paths to data files
paths = [
f"../_static/section-04/isothermal-properties-for-{species}-Tr={T_r}.csv"
for species in ["argon", "oxygen", "water", "carbon-dioxide"]
for T_r in [1.0, 1.2, 2.0]
]
# Create figure
fig, ax = plt.subplots(figsize=(4, 4))
# Load and plot data
for i, path in enumerate(paths):
df = pd.read_csv(path)
df.rename(columns={"Temperature (K)": "T", "Pressure (bar)": "P_bar", "Volume (m3/mol)": "Vm"}, inplace=True)
df["P"] = df["P_bar"] * 1e5 # Convert bar to Pa
df["Z"] = df["P"] * df["Vm"] / (R * df["T"])
species = df["substance"].values[0]
T_r = df["Tr"].values[0].round(1)
color = color_for_temperature[T_r]
linestyle = line_style_for_species[species]
label = f"$T_r = {T_r}$" if i % 4 == 0 else None
line = ax.plot(df["Pr"], df["Z"], color=color, linestyle=linestyle, alpha=0.5, label=label)
if label:
labelLines(line, align=False, xvals=[2], zorder=2.5)
# Create legend
legend_elements = [Line2D([0], [0], c='k', label=label_for_species[s], ls=line_style_for_species[s], alpha=0.5)
for s in line_style_for_species]
ax.legend(handles=legend_elements, loc='lower right')
# Set labels and grid
ax.set_xlim(0, 8)
ax.set_ylim(0, 1.25)
ax.set_xlabel("Reduced Pressure ($P_r = P / P_c$)")
ax.set_ylabel("$Z$")
ax.grid(True, linestyle="--", alpha=0.7)
plt.tight_layout()
glue('corresponding-states', fig, display=False)
plt.close(fig)
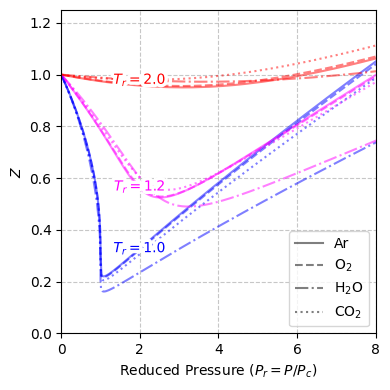
Fig. 11 Compressibility factor \(Z\) of argon, oxygen, water, and carbon dioxide plotted over a range of reduced pressures for different reduced temperatures. These data illustrate how real gases exhibit similar trends when expressed in terms of their critical properties, in accordance with the principle of corresponding states.#
Redefining \(V_\text{m}\), \(P\), and \(T\) as fractions \(V_{\text{m},r}\), \(P_r\), and \(T_r\) of their critical values:
and substituting these definitions into Equation (8) reveals a significant result:
This result—called the principle of corresponding states—illustrates that the equations of state for all van der Waals fluids are alike when expressed relative to their critical properties.
Tip
Calculate the value of the compressibility factor for a van der Waals fluid at the critical point? Do you notice anything interesting?
Complete Derivation of the Corresponding States Equation
1. Substitute the Definitions of \(V_\text{m}\), \(P\), and \(T\) Into Equation (8):
2. Divide Both Sides by \(P_c V_{\text{m},c}\):
3. Substitute the Definitions of \(P_c\), \(V_{\text{m},c}\), and \(T_c\) In Terms of \(a_\text{m}\), \(b_\text{m}\), and \(R\):
