1. Course Introduction#
1.1. Overview#
This course covers the fundamental concepts of thermodynamics and statistical mechanics, along with their applications to chemical systems. It builds upon the thermodynamic principles introduced in Chem 106 and Chem 112, and the quantum mechanics taught in Chem 105, Chem 111, and Chem 401. By connecting molecular-level behavior to macroscopic thermodynamic observations, students will learn how theory underpins real-world chemical processes.
1.2. Why Study Thermodynamics and Statistical Mechanics?#
1.2.1. Bridging the Microscopic and Macroscopic Worlds#
Typical chemical systems contain on the order of Avogadro’s number of particles (i.e., \(N_\text{A} = 6.022 \times 10^{23}\)). Thermodynamics abstracts the complexity of such large collections of molecules into a framework for predicting:
Reaction spontaneity,
Equilibrium states,
Phase transitions, and more.
1.2.2. Modern Applications#
Thermodynamics and statistical mechanics are central to diverse fields, including industrial chemistry, materials science, and biochemistry. The examples below highlight just a few applications:
1.3. Key Definitions#
1.3.1. Thermodynamic Systems#
Show code cell source
import matplotlib.pyplot as plt
import matplotlib.patches as mpatches
from myst_nb import glue
# Helper function to plot a system
def plot_system(ax, title, annotations, boundary_color='b'):
box = mpatches.FancyBboxPatch((0, 0), 1, 1, boxstyle='roundtooth', ec=boundary_color, fc='w')
ax.add_patch(box)
ax.set_title(title, fontsize=14)
ax.text(0.5, 0.5, 'System', ha='center', va='center', fontsize=12)
ax.text(0.5, -0.65, 'Surroundings', ha='center', va='center', fontsize=12)
ax.text(0.5, 1.3, 'Boundary', ha='center', va='bottom', fontsize=12, color=boundary_color)
for annotation in annotations:
if "arrowprops" in annotation: # Arrow annotations
ax.annotate('', **annotation)
else: # Text annotations
ax.text(**annotation)
ax.set_xlim(-1, 2)
ax.set_ylim(-1, 2)
ax.set_aspect('equal')
ax.axis('off')
# Define annotations for each system
annotations = [
[], # Isolated system (no arrows)
[ # Closed system (energy arrow)
dict(xy=(-0.6, 0.15), xytext=(0.15, 0.15), arrowprops=dict(arrowstyle='<->', color='r')),
dict(x=-1, y=0.3, s='Energy', ha='left', va='bottom', fontsize=12, color='r'),
],
[ # Open system (energy + matter arrows)
dict(xy=(-0.6, 0.15), xytext=(0.15, 0.15), arrowprops=dict(arrowstyle='<->', color='r')),
dict(xy=(0.85, 0.15), xytext=(1.6, 0.15), arrowprops=dict(arrowstyle='<->', color='m')),
dict(x=-1, y=0.3, s='Energy', ha='left', va='bottom', fontsize=12, color='r'),
dict(x=2, y=0.3, s='Matter', ha='right', va='bottom', fontsize=12, color='m'),
],
]
titles = ["Isolated system", "Closed system", "Open system"]
fig, axes = plt.subplots(1, 3, figsize=(12, 4))
for i, ax in enumerate(axes):
plot_system(ax, titles[i], annotations[i])
glue('thermo-systems', fig, display=False)
plt.close(fig)
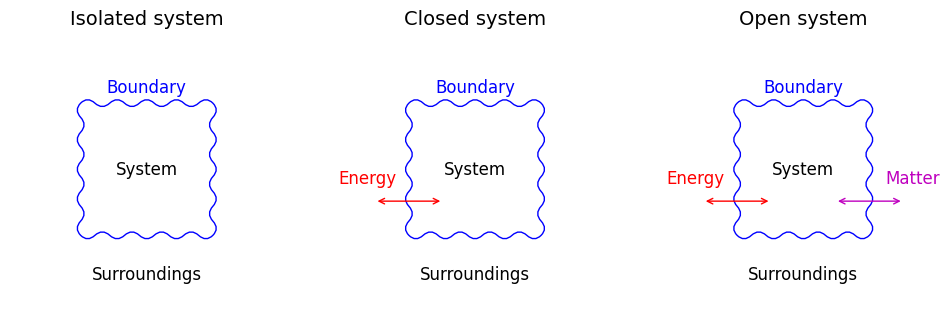
Fig. 1.4 Types of thermodynamic systems: (a) Isolated—no exchange of energy or matter; (b) Closed—exchanges energy but not matter; and (c) Open—exchanges both energy and matter.#
- System#
The portion of the universe chosen for study, separated from its surroundings by a boundary.
- Surroundings#
Everything external to the system that can exchange energy or matter with it.
- Boundary#
The interface separating a system from its surroundings.
- Isolated system#
Exchanges neither energy nor matter with its surroundings.
- Closed system#
Exchanges energy but not matter with its surroundings.
- Open system#
Exchanges both energy and matter with its surroundings.
1.3.2. State of a System#
---
config:
flowchart:
htmlLabels: false
layout: elk
look: handDrawn
theme: neutral
---
flowchart TB
subgraph B["Microscopic"]
direction TB
B1["Classical"] ~~~ B2["Quantum"]
end
subgraph C["Macroscopic"]
direction TB
C1["Equilibrium"]
end
subgraph C1["Equilibrium"]
direction TB
C11["Thermodynamic"]
end
subgraph C11["Thermodynamic"]
direction LR
C111["State variables"] ~~~ C112["State functions"] & C113["Path functions"]
end
subgraph C111["State variables"]
direction TB
C1111["Extensive"] ~~~ C1112["Intensive"]
end
A["State of a system"] --> B & C
- Particle#
A microscopic entity such as an atom, molecule, or ion.
- Microscopic state (classical)#
Positions and momenta of all particles in the system.
- Microscopic state (quantum)#
The wavefunction describing the system’s particles.
- Equilibrium#
A condition in which macroscopic properties remain constant over time.
- Thermodynamic equilibrium#
A state of simultaneous mechanical, thermal, and chemical equilibrium.
- Thermodynamic state#
A set of macroscopic variables defining a system in equilibrium.
- State variable#
A property that defines a system’s state.
- State function#
A property depending only on the system’s state, not on the path taken.
- Equation of state#
A mathematical relationship among state variables.
- Path function#
A property depending on the process or path taken between states.
- Process#
A transformation changing a system from one state to another.
- Extensive property#
A property proportional to system size (e.g., volume, entropy).
- Intensive property#
A property independent of system size (e.g., temperature, pressure).
1.4. Basic Forms of Energy and Energy Transfer#
---
config:
flowchart:
htmlLabels: false
layout: elk
look: handDrawn
theme: neutral
---
flowchart TB
subgraph H1["Electrostatic"]
direction TB
H11["Charge–charge"] ~~~ H12["Charge–dipole"]
end
subgraph H2["van der Waals"]
direction TB
H21["Dipole–dipole"] ~~~ H22["Induced dipole"] ~~~ H23["Dispersion"]
end
subgraph H["Electric"]
direction LR
H1 ~~~ H2
end
subgraph I1["Intramolecular"]
direction TB
I11["Covalent"] ~~~ I12["Ionic"] ~~~ I13["Metallic"]
end
subgraph I2["Intermolecular"]
direction TB
I21["Hydrogen"] ~~~ I22["van der Waals"]
end
subgraph I["Chemical"]
direction LR
I1 ~~~ I2
end
subgraph J["Mechanical"]
direction TB
J1["Expansion"] ~~~ J2["Surface expansion"] ~~~ J3["Extension"]
end
subgraph G["Work"]
direction LR
J ~~~ G1["Electrical"]
end
A["Basic forms of energy and energy transfer"] --> B["Energy"] & C["Energy transfer"]
B --> D["Kinetic"] & E["Potential"]
C --> F["Heat"] & G
E --> H & I
H1 & H2
I1 & I2
style H11 stroke:red, stroke-width:2px
style H2 stroke:blue, stroke-width:2px
style I12 stroke:red, stroke-width:2px
style I22 stroke:blue, stroke-width:2px
1.4.1. Energy#
1.4.2. Energy Transfer#
1.5. Important Units#
1.5.1. SI Units#
Quantity |
Unit |
Symbol |
|---|---|---|
Time |
second |
s |
Length |
meter |
m |
Mass |
kilogram |
kg |
Temperature |
kelvin |
K |
Quantity |
Unit |
Symbol |
Conversion |
|---|---|---|---|
Frequency |
hertz |
Hz |
\(1 \,\text{Hz} = 1 \,\text{s}^{-1}\) |
Force |
newton |
N |
\(1 \,\text{N} = 1 \,\text{kg m s}^{-2}\) |
Pressure |
pascal |
Pa |
\(1 \,\text{Pa} = 1 \,\text{N m}^{-2}\) |
Energy |
joule |
J |
\(1 \,\text{J} = 1 \,\text{N m}\) |
1.5.2. Non-SI Units#
Quantity |
Unit |
Symbol |
Conversion |
|---|---|---|---|
Pressure |
bar |
bar |
\(1 \,\text{bar} = 1 \times 10^5 \,\text{Pa}\) |
atmosphere |
atm |
\(1 \,\text{atm} \approx 1.01325 \,\text{bar}\) |
|
torr |
torr |
\(1 \,\text{torr} = \frac{1}{760}\,\text{atm}\) |
|
millimeters of mercury |
mmHg |
\(1 \,\text{mmHg} = 1 \,\text{torr}\) |
|
Energy |
electronvolt |
eV |
\(1 \,\text{eV} = 1.602 \times 10^{-19} \,\text{J}\) |
calorie |
cal |
\(1 \,\text{cal} = 4.184 \,\text{J}\) |